Understanding Electrovalent Bonds: Formation, Types, Properties, and Examples of lewis dot structure
Examples of Electrovalent Bond Formation
Introduction:
Electrovalent bonds, also known as ionic bonds, are one of the most common types of chemical bonds in chemistry.
Definition:
They are formed when atoms of different elements transfer electrons to each other, resulting in the formation of oppositely charged ions.The ions formed are attracted to each other, creating a stable compound through electrostatic forces.
Importance:
Electrovalent bonds are important in chemistry because they explain the properties and behaviors of many substances, such as salts, metals, and minerals. In this blog post, we will explore what electrovalent bonds are, how they are formed, and some examples of electrovalent bond formation.
What is an Electrovalent Bond?
Definition of Electrovalent Bond
An electrovalent bond results from the transfer of one or more electrons from one atom to another.
The atom that loses electrons becomes a positively charged ion, called a cation, while the atom that gains electrons becomes a negatively charged ion, called an anion. The cation and anion are held together by electrostatic attraction due to their opposite charges, also known as an ionic or salt bond.
Difference between Electrovalent and Covalent Bond
Covalent bonds involve the sharing of one or more pairs of electrons between two atoms.The atoms that share electrons form a molecule, which is a neutral group of atoms. A covalent bond is also called a molecular bond or a non-ionic bond.
The main difference between electrovalent and covalent bonds is that electrovalent bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons.
Another difference is that electrovalent bonds form between atoms of different electronegativities, while covalent bonds form between atoms of similar electronegativities.
Electronegativity measures how strongly an atom attracts electrons in a bond.
Higher the electronegativity difference between two atoms, the more likely they are to form an electrovalent bond.
How are Electrovalent Bonds Formed?
Factors Affecting Electrovalent Bond Formation
The formation of electrovalent bonds depends on several factors, such as the size, charge, and energy of the ions involved. Generally, electrovalent bonds are more likely to form when:
The cation is small and has a high charge, which means it has a high ionization energy. Ionization energy is the energy required to remove an electron from an atom.
high ionization energy means that the cation can easily lose electrons and become stable.
The anion is large and has a low charge, which means it has a high electron affinity.Electron affinity is the energy released when an atom gains an electron.
A high electron affinity means that the anion can easily gain electrons and become stable.
The compound's lattice energy is high, signifying the energy released when oppositely charged ions form a solid crystal lattice.
A high lattice energy means that the compound is very stable and has a low melting point.
Lewis Dot Structure of Electrovalent Bonds
A Lewis dot structure is a way of representing the valence electrons (the outermost electrons) of atoms and molecules using dots and lines. Valence electrons are important because they determine how atoms bond with each other.
To draw a Lewis dot structure for an electrovalent compound, follow these steps:
- Write the symbols of the cation and the anion next to each other.
- Represent the valence electrons by encircling each symbol using dots. Utilize the periodic table to determine the count of valence electrons for each element.
- Draw arrows to show how the electrons are transferred from the cation to the anion. The number of arrows should match the charge of each ion.
- Enclose each ion in brackets and write its charge as a superscript.
- Check that each ion has a complete octet (eight valence electrons) or duet (two valence electrons) after the transfer.
Examples of Electrovalent Bond Formation
Here are some examples of electrovalent bond formation between different elements:
Sodium Chloride (NaCl)
An exemplary illustration is sodium chloride, commonly known as table salt, which forms as an electrovalent compound through the transfer of one electron from sodium (Na) to chlorine (Cl).
Sodium is a metal with one valence electron, while chlorine is a nonmetal with seven valence electrons. Sodium has a low ionization energy and chlorine has a high electron affinity, which means they can easily form an electrovalent bond. The resulting compound has a high lattice energy and a low melting point.
The chemical equation for the formation of sodium chloride is:
Na + Cl → Na+ + Cl- → NaCl
The Lewis dot structure for sodium chloride is:
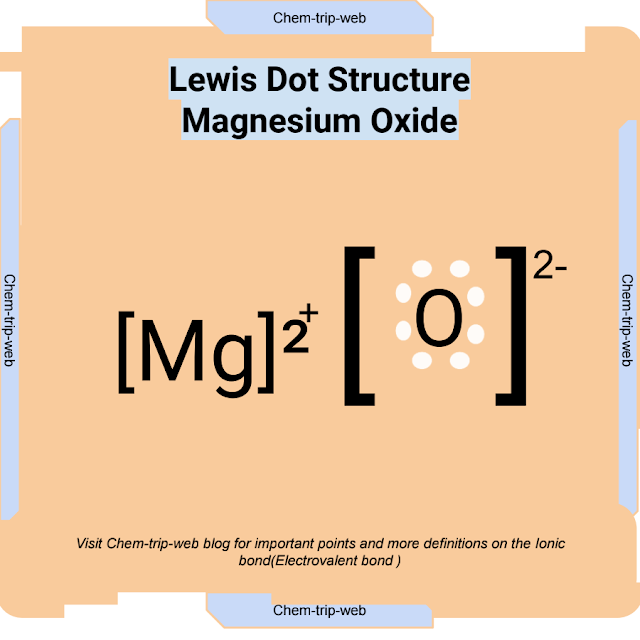
Magnesium Oxide (MgO)
Magnesium oxide, also known as magnesia, is an electrovalent compound formed by the transfer of two electrons from magnesium (Mg) to oxygen (O). Magnesium is a metal with two valence electrons, while oxygen is a nonmetal with six valence electrons. Magnesium has a high ionization energy and oxygen has a high electron affinity, which means they can form a strong electrovalent bond. The resulting compound has a very high lattice energy and a very high melting point.
The chemical equation for the formation of magnesium oxide is:
Mg + O → Mg2+ + O2- → MgO
The Lewis dot structure for magnesium oxide is:
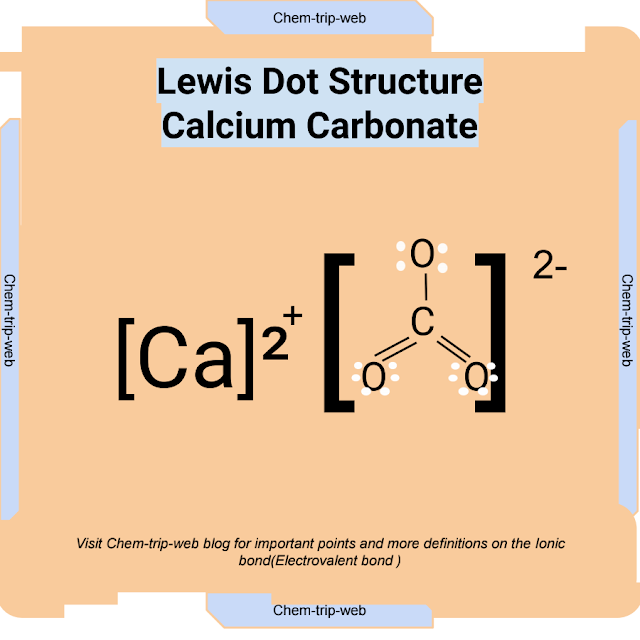
Calcium Carbonate (CaCO3)
Calcium carbonate, also known as limestone or chalk, is an electrovalent compound formed by the combination of calcium (Ca) and carbonate (CO3) ions. Calcium is a metal with two valence electrons, while carbonate is a polyatomic ion (a group of atoms that act as a single unit) with two negative charges. Calcium has a high ionization energy and carbonate has a high electron affinity, which means they can form an electrovalent bond. The resulting compound has a moderate lattice energy and a moderate melting point.
The chemical equation for the formation of calcium carbonate is:
Ca + CO3 → Ca2+ + CO3 2- → CaCO3
The Lewis dot structure for calcium carbonate is:
Properties of Electrovalent Compounds
Electrovalent compounds have some distinctive physical and chemical properties that are different from covalent compounds. Here are some of the common properties of electrovalent compounds:
Physical Properties
- Electrovalent compounds are usually solid at room temperature and have high melting and boiling points. Due to the formidable electrostatic attraction between ions, requiring substantial energy to overcome.
- Electrovalent compounds are generally hardness and brittle. This is because the ions are arranged in a rigid crystal lattice that can break easily when subjected to stress or pressure.
- Electrovalent compounds display excellent electrical conductivity in their molten or dissolved state in water.
- This is because the ions are free to move and carry electric charges when they are in liquid or aqueous state.
- Electrovalent compounds are soluble in polar solvents, such as water, but insoluble in non polar solvents, such as oil. This is because polar solvents can dissolve the ions by forming intermolecular forces with them, while non polar solvents cannot.
Chemical Properties
- Electrovalent compounds tend to undergo displacement reactions with metals or nonmetals that have higher or lower electronegativities respectively. This is because the ions can exchange electrons with other atoms that have more or less tendency to gain or lose electrons respectively.
- Electrovalent compounds tend to undergo decomposition reactions when heated or exposed to light or electricity. This is because the ions can break apart into simpler substances when they receive enough energy from external sources.
- Electrovalent compounds tend to form precipitates when mixed with other solutions that contain ions of opposite charges. This is because the ions can combine with each other to form insoluble solids that settle at the bottom of the container.
Conclusion
Electrovalent bonds are one of the most important types of chemical bonds in chemistry. They are formed when atoms of different elements transfer electrons to each other, resulting in the formation of oppositely charged ions. The ions formed are attracted to each other, creating a stable compound through electrostatic forces.
Electrovalent bonds explain the properties and behaviors of many substances,

Comments
Post a Comment